
Thus, the molar mass of a substance is the mass in grams of 1.
#Molar mass of hydrogen mac#
Our “mole ratio”, so to speak, for milk and mac n’ cheese is 1 mac n’ cheese over 3 tablespoons of milk. mass of 1 mole of methane molecules, it makes sense to call it the molar mass for methane. One box of pasta, 1 packet of cheese, 3 tablespoons of milk, and 2 tablespoons of butter.ġ box pasta + 1 packet of cheese + 3 tbsp milk + 2 tbsp butter = 1 mac n’cheese For Lithium, there are two isotopes: Lithium. 2) Determine the mass of hydrogen present in one mole of water: In one mole of water, there are two moles of H atoms (2 mol) (1.008 g/mol) 2.016 g. The atomic weight is determined by taking the known abundance and mass of each isotope and solving for the average.
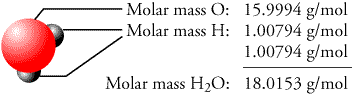
When you make mac n’ cheese, you always use the same ratio of ingredients. Determine the molar mass of water: one mole of water weighs 18.0152 grams. This is most certainly a concept best learned through examples, so let’s jump right in.īefore we put these mole ratios to use, let’s consider a more relatable example to solidify this concept. Among other things, it’s necessary for calculating theoretical yields and limiting reagents. It’s a fundamental concept in chemistry that’s used to convert back and forth between moles of each substance in a reaction. The entered volume of Hydrogen in various units of volume Molecular formula: H Molecular weight: 2.016 g/mol Molar volume: 24.585 dm/mol CAS Registry. Thus, the formula mass of calcium hydrogen carbonate is 117.10 amu and the molar mass of calcium hydrogen carbonate is 117.10 grams per mole (g/mol). We can see that the ideal gas law is suitable up to 100 bar and that, at higher pressures, the viral equation predicts a higher work demand.
Also, the ideal gas specific compression work is plotted, dashed black line. However when talking about a mole of an ionic compound we will still use the term molar mass. (8.22) divided by the molar mass of hydrogen is plotted as a function of pressure in Fig. Hi, and welcome to this video on the mole ratio, sometimes referred to as the molar ratio. This is because there are no individual molecules in ionic compounds.


 0 kommentar(er)
0 kommentar(er)
